Atoms
Atoms
All elements consists of very small invisible particles, called atom. Every atom is a sphere of radius of the order of 10-10 m, in which entire mass is uniformly distributed and negative charged electrons revolve around the nucleus.
Thomson’s Model of an Atom:
An atom consists of positively charged matter in which the negatively charged electrons are uniformly embedded like plums in a pudding. This model could not explain scattering of alpha-particles through thin foils and hence discarded.
Rutherford’s Model of an Atom:
Basic assumption of Rutherford’s atomic model:(i) Atom consists of small central core, called atomic nucleus in which whole mass and positive charge is assumed to be concentrated.
(ii) The size of nucleus is much smaller than the size of the atom.
(iii) The nucleus is surrounded by electrons and atom is electrically neutral.

From the results of these experiments, Rutherford proposed the following model of an atom:-
a) An atom consists of a small and massive central core in which the entire positive charge and almost the whole mass of the atom are concentrated. This core is called the nucleus.
b) The nucleus occupies a very small space as compared to the size of the atom.
c) The atom is surrounded by a suitable number of electros so that their total negative charge is equal to the total positive charge on the nucleus and the atom as a whole is electrically neutral.
d) The electrons revolve around the nucleus in various orbits just as planets revolve around the sun.
e) The centripetal force required for their revolution is provided by the electrostatic attraction between the electrons and the nucleus.
2. Experimental arrangement for α-scattering experiment and trajectory followed by α -particles


Draw-back of Rutherford Model:
(i) It could not explained stability of atom clearly.
(ii) It was unable to explain line spectrum of Hydrogen atom.
Distance of Closest Approach:
Hence,
Impact Parameter:
a) It is defined as the perpendicular distance of the velocity of the alpha-particle from the centre of the nucleus, when it is far away from the atom.
b) The shape of the trajectory of the scattered alpha-particle depends on the impact parameter b and the nature of the potential field.
c) Rutherford deduced the following relationship between the impact parameter b and the scattering angle :-

Quantisation or Discretisation:
Bohr’s Model for the Hydrogen Atom:

Basic postulates:-
a) Nuclear concept:
An atom consists of a small massive centre called nucleus around which planetary electrons revolve. The centripetal force required for their rotation is provided by the electrostatic attraction between the electrons and the nucleus.
b) Quantum condition:
Of all the possible circular orbits allowed by the classical theory, the electrons are permitted to circulate only in such orbits in which the angular momentum of an electron is an integral multiple of , h being Planck’s constant.
where n is called principal quantum number.
c) Stationary orbits:
While revolving in the permissible orbits, an electron does not radiate energy. These non-radiating orbits are called stationary orbits.
d) Frequency condition:
An atom can emit or absorb radiation in the form of discrete energy photons only when an electron jumps from a higher to a lower orbit or from a lower to a higher orbit. If E1 and E2 are the energies associated with these permitted orbits then the frequency of the emitted/absorbed radiation is,
e) Radius of the orbit of an electron in hydrogen atom is,
f) Kinetic energy K & electrostatic potential energy U of the electron in hydrogen atom:-
g) Total energy E of the electron in hydrogen atom:-
h) Speed of an electron in the nth orbit is,
Where
is fine structure constant.
i) Energy of an electron in nth orbit is,
Failure of Bohr’s Model:
a) This model is applicable only to hydrogen-like atoms and fails in case of higher atoms.
b) It could not explain the fine structure of the spectral lines in the spectrum of hydrogen atom.
Energy Level Diagram:
Different Spectral Series of Hydrogen Atom:
These are as follows:
Hydrogen spectrum contains five series, namely:
(i) Lyman Series
When electron jumps from an orbit with n = 2, 3,4, …orbit to the first orbit with n = 1 orbit, then a line of Lyman series is obtained.
This series lies in ultra violet region.
(ii) Balmer Series
When electron jumps from an orbit with n = 3, 4, 5,… orbit to the first orbit with n = 2 orbit, then a line of Balmer series is obtained.
This series lies in visual region.
(iii) Paschen Series
When electron jumps from an orbit with n = 4, 5, 6,… orbit to the first orbit with n = 3 orbit, then a line of Paschen series is obtained.
This series lies in infrared region
(iv) Brackett Series
When electron jumps from an orbit with n = 5,6, 7…. orbit to the first orbit with n = 4 orbit, then a line of Brackett series is obtained.
This series lies in infrared region.
(v) Pfund Series
When electron jumps from an orbit with n = 6,7,8, … orbit to the first orbit with n = 5 orbit, then a line of Pfund series is obtained.
This series lies in infrared region.
Wave Model
It is based on wave mechanics. Quantum numbers are the numbers required to completely specify the state of the electrons.
In the presence of strong magnetic field, the four quantum numbers are
(i) Principal quantum number (n) can have value 1,2, … ∞
(ii) Orbital angular momentum quantum or Azimuthal quantum number ( ℓ ) can have value 0,1, 2, … ,(n – 1).
(iii) Magnetic quantum number (me) which can have values – I to I.
(iv) Magnetic spin angular momentum quantum number ( m s ) which can have only two value + 1 / 2.
Electron Orbitals and their Shape:
Excitation Energy:
Excitation Potential:
Ionisation Potential:
De Broglie’s Hypothesis:
The concept that matter behaves like wave is called the de Broglie hypothesis, named after Louis de Broglie, who proposed it in 1924.
The electrons having a wavelength
de Broglie gave the following equation which can be used to calculate de Broglie wavelength, , of any massed particle whose momentum is known:
where is the Plank's constant and is the momentum of the particle whose wavelength we need to find.
With some modifications the following equation can also be written for velocity or kinetic energy of the particle (of mass ):
MASER:-
a) Maser stands for ‘Microwave Amplification by Stimulated Emission of Radiation’.
b) It is simply a device for producing a highly intense, monochromatic coherent and collimated beam of microwaves.
LASER:-
a) It stand for ‘Light Amplification by Stimulated Emission of Radiation.
b) It is a device used to produce highly intense strong monochromatic coherent and collimated beam of light.
Problem :
Calculate the de Broglie wavelength of a golf ball whose mass is 40 grams and whose velocity is 6 m/s.
We have
Home Work :
Level – 1
1. Calculate the longest wavelength in the Paschen series of He+ ion.
2. Calculate the ratio of the wavelength of first and the ultimate line of Balmer series of Li2+ .
3 Calculate the number of photons emitted in 10 hours by a 60 W sodium lamp (λ = 5893 A °).
4. An electron collides with a hydrogen atom in its ground state and excited it to a state of n = 3. How much energy was given to the hydrogen atom in this inelastic collision ?
5. Hydrogen atom in states of high quantum number have been created in the laboratory and observed in space.
a) find the quantum number of the Bohr orbit in a H-atom whose radius is 0.0100 mm ?
b) what is the energy of H-atom in this state?
6. With what velocity should an α-particle travel towards the nucleus of a copper atom so as to arrive at a distance 10-13 metre from the nucleus of the copper atom.
7. An electron beam can undergo diffraction by crystals. Through what potential should a beam of electrons be accelerated. So that its wave length becomes equal to 1.54 A ° .
Chapter at a glance
1. Dalton’s Atomic Theory
All elements are consists of very small invisible particles, called atoms. Atoms of same element are exactly same and atoms of different element are different.
2. Thomson’s Atomic Model
Every atom is uniformly positive charged sphere of radius of the order of 10-10 m, in which entire mass is uniformly distributed and negative charged electrons are embedded randomly. The atom as a whole is neutral.

Limitations of Thomson’s Atomic Model
1. It could not explain the origin of spectral series of hydrogen and other atoms.
2. It could not explain large angle scattering of α – particles.
3. Rutherford’s Atomic Model
On the basis of this experiment, Rutherford made following observations
(i) The entire positive charge and almost entire mass of the atom is concentrated at its centre in a very tiny region of the order of 10-15 m, called nucleus.
(ii) The negatively charged electrons revolve around the nucleus in different orbits.
(iii) The total positive charge 011 nucleus is equal to the total negative charge on electron. Therefore atom as a overall is neutral.
(iv) The centripetal force required by electron for revolution is provided by the electrostatic force of attraction between the electrons and the nucleus.
4. Limitations of Rutherford Atomic Model
(i) About the Stability of Atom According to Maxwell’s electromagnetic wave theory electron should emit energy in the form of electromagnetic wave during its orbital motion. Therefore. radius of orbit of electron will decrease gradually and ultimately it will fall in the nucleus.
(ii) About the Line Spectrum Rutherford atomic model cannot explain atomic line spectrum.
Impact parameter :
It is the perpendicular distance of the velocity vector of a-particle from the central line of the nucleus of the atom is called impact parameter (b).where, K is KE of α-particle, θ is scattering angle, Z is atomic number of the nucleus and e is charge of nucleus.
The perpendicular distance of the velocity vector of a-particle from the central line of the nucleus, when the particle is far away from the nucleus is called impact parameter.

5. Distance of Closest Approach At a certain distance r0 from the nucleus, whole of the KE of α-particle converts into electrostatic potential energy and α-particle cannot go farther close to nucleus, this distance (r0) is called distance of closest approach.
ro = 1 / 4π εo . 2Ze2 / Ek
where, Ek = kinetic energy of the cc-particle.
6. Angle of Scattering Angle by which a-particle gets deviated from its original path around the nucleus is called angle of scattering.
7. Rutherford’s Scattering Formula

where, N(θ) =number of c-particles, Ni = total number of α-particles reach the screen. n = number of atoms per unit volume in the foil, Z = atoms number, E = kinetic energy of the alpha particles and t = foil thickness
Bohr’s Theory of Hydrogen Atom
Electron can revolve in certain non-radiating orbits called stationary or bits for which the angular momentum of electron is an integer multiple of (h / 2π)
mvr = nh / 2π
where n = I, 2. 3,… called principal quantum number.
The radiation of energy occurs only when any electron jumps from one permitted orbit to another permitted orbit.
Energy of emitted photon
hv = E2 – E1
where E1 and E2are energies of electron in orbits.
Radius of orbit of electron is given by
r = n2h2 / 4π2 mK Ze2 ⇒ r ∝ n2 / Z
where, n = principle quantum number, h = Planck’s constant, m = mass of an electron, K = 1 / 4 π ε, Z = atomic number and e = electronic charge.
Velocity of electron in any orbit is given by
v = 2πKZe2 / nh ⇒ v ∝ Z / n
Frequency of electron in any orbit is given by
v = KZe2 / nhr = 4π2Z2e4mK2 / n3 h3
⇒ v prop; Z3 / n3
Kinetic energy of electron in any orbit is given by
Ek = 2π2me4Z2K2 / n2 h2 = 13.6 Z2 / n2 eV
Potential energy of electron in any orbit is given by
Ep = – 4π2me4Z2K2 / n2 h2 = 27.2 Z2 / n2 eV
⇒ Ep = ∝ Z2 / n2
Total energy of electron in any orbit is given by
E = – 2π2me4Z2K2 / n2 h2 = – 13.6 Z2 / n2 eV
⇒ Ep = ∝ Z2 / n2
Wavelength of radiation emitted in the radiation from orbit n2 to n1 is given by

In quantum mechanics, the energies of a system are discrete or quantized. The energy of a particle of mass m is confined to a box of length L can have discrete values of energy given by the relation
En = n2 h2 / 8mL2 ; n < 1, 2, 3,…
The Concept of Bohr's Theory
8. Bohr’s Theory of Hydrogen Atom
Bohr combined classical and early quantum concepts and gave his theory in the form of three postulates. These are
(i) Bohr’s first postulate was that an electron in an atom could revolve in certain stable orbits without the emission of radiant energy, contrary to the predictions of electromagnetic theory.
(ii) Bohr’s second postulate defines these stable orbits. This postulate states that the electron revolves around the nucleus only in those orbits for which the angular momentum is some integral multiple of h/2π, where h is the Planck’s constant (= 6.6 x 10-34 J – s). Thus, the angular momentum (L) of the orbiting electron is
quantised, i. e. L = nh/2π
As, angular momentum of electron (L) = mvr
∴ For any permitted (stationary) orbit, mvr = nh/2π
where, n = any positive integer i.e. 1, 2, 3, ….
It is also called principal quantum number.
(iii) Bohr’s third postulate states that an electron might make a transition from one of its specified non-radiating orbits to another of lower energy. When it does so, a photon is emitted having energy equal to the energy difference between the initial and final states.
The frequency of the emitted photon is then given by
hv – Ei– Ef
where, Ei and Ef are the energies of the initial and final states and Ei > Ef .
9. Limitations of Bohr’s Model
(i) Applicable only for hydrogen like atom.
(ii) Does not explain the fine structure of spectral lines in H-atom.
(iii) Does not explain about shape of orbit.
10. Important formulae related to Bohr’s model of hydrogen atoms are given below:
11. Energy Level The energy of an atom is the least when its electron is revolving in an orbit closest to the nucleus i.e. for which n = 1.
12. The lowest state of the atom is called the ground state, this state has lowest energy. The energy of this state is -13.6 eV. Therefore, the minimum energy required to free the electron from the ground state of the hydrogen atom is -13.6 eV.
13. (i) Emission Spectrum Hydrogen spectrum consists of discrete bright lines a dark
background and it is specifically known as hydrogen emission spectrum.
(ii) Absorption Spectrum There is one more type of hydrogen spectrum exists where we get dark lines on the bright background, it is known as absorption spectrum
14. The atomic hydrogen emits a line spectrum consisting of various series.
Some Solved Examples:
EXAMPLE 12.1
The radius of the 5th orbit of hydrogen atom is 13.25 Å. Calculate the wavelength of the electron in the 5th orbit.
Solution:
2πr = nλ
2 × 3.14 × 13.25Å = 5 × λ
λ = 16.64Å
EXAMPLE 12.2
Find the (i) angular momentum (ii) velocity of the electron in the 5th orbit of hydrogen atom. (h = 6.6 × 10–34 Js, m = 9.1 × 10–31 kg)
Solution
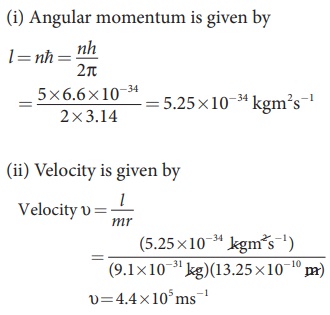
EXAMPLE 12.3
(a) Show that the ratio of velocity of an electron in the first Bohr orbit to the speed of light c is a dimensionless number.
(b) Compute the velocity of electrons in ground state, first excited state and second excited state in Bohr atom model.
Solution
(a) The velocity of an electron in nth orbit is
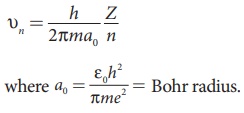
Substituting for a0 in υn,
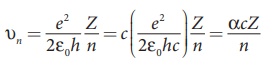
where c is the speed of light in free space or vacuum and its value is c = 3 × 108 m s–1and α is called fine structure constant.
For a hydrogen atom, Z = 1 and for the first orbit, n = 1, the ratio of velocity of electron in first orbit to the speed of light in vacuum or free space is
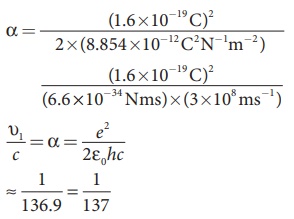
which is a dimensionless number
⇒ α = 1/137
(b) Using fine structure constant, the velocity of electron can be written as
υn = αcZ / n
For hydrogen atom (Z = 1) the velocity of electron in nth orbit is
υn = c/137 × 1/n = (2.19×106) × 1/n ms−1
For the first orbit (ground state), the velocity of electron is
υ = 2.19×106 ms−1
For the second orbit (first excited state), the velocity of electron is
υ2 = 1.095×106 ms−1
For the third orbit (second excited state), the velocity of electron is
υ3 = 0.73×106 ms−1
Here, υ1 > υ2 > υ3
EXAMPLE 12.4
The Bohr atom model is derived with the assumption that the nucleus of the atom is stationary and only electrons revolve around the nucleus. Suppose the nucleus is also in motion, then calculate the energy of this new system.
Solution
Let the mass of the electron be m and mass of the nucleus be M. Since there is no external force acting on the system, the centre of mass of hydrogen atom remains at rest. Hence, both nucleus and electron move about the centre of mass as shown in figure.
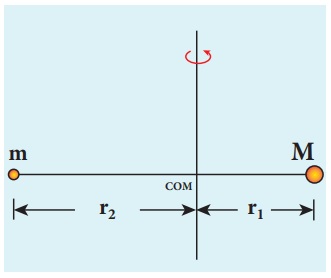
Let V be the velocity of the nuclear motion and υ be the velocity of electron motion. Since the total linear momentum of the system is zero,
−mυ + Mυ = 0 or
MV = mυ = p
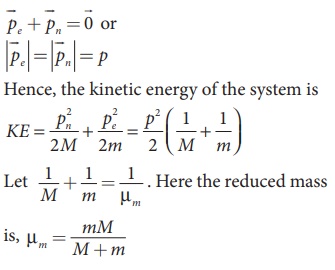
Since the potential energy of the system is same, the total energy of the hydrogen can be expressed by replacing mass by reduced mass, which is
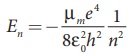
Since the nucleus is very heavy compared to the electron, the reduced mass is closer to the mass of the electron.
EXAMPLE 12.5
Suppose the energy of a hydrogen–like atom is given as En = − 54.4/n2 eV where n∈N . Calculate the following:
(a) Sketch the energy levels for this atom and compute its atomic number.
(b) If the atom is in ground state, compute its first excitation potential and also its ionization potential.
(c) When a photon with energy 42 eV and another photon with energy 56 eV are made to collide with this atom, does this atom absorb these photons?
(d) Determine the radius of its first Bohr orbit.
(e) Calculate the kinetic and potential energies in the ground state.
Solutions
(a) Given that
En =− 54.4/n2 eV
For n = 1, the ground state energy E1= –54.4 eV and for n = 2, E2 = –13.6 eV.
Similarly, E3 = –6.04 eV, E4 = –3.4 eV and so on.
For large value of principal quantum number – that is, n = ∞, we get E∞ = 0 eV.

(b) For a hydrogen-like atom, ground state energy is
E1 = − 13.6/n2 Z2 eV
where Z is the atomic number. Hence, comparing this energy with given energy, we get, – 13.6 Z2 = – 54.4 ⇒ Z = ±2. Since, atomic number cannot be negative number, Z = 2.
(c) The first excitation energy is
EI = E2 − E1 = −13.6eV −(−54.4 eV)
= 40.8eV
Hence, the first excitation potential is
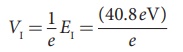
= 40.8 volt
The first ionization energy is
Eionization = E∞ − E1 = 0 −(−54.4 eV)
= 54.4 eV
Hence, the first ionization potential is
Vionization = 1/e Eionization = (54.4eV)/e
= 54.4 Volt
(d) Consider two photons to be A and B.
Given that photon A with energy 42 eV and photon B with energy 51 eV
From Bohr assumption, difference in energy levels is equal to photon energy, then atom will absorb energy, otherwise, not.
E2 − E1 = −13.6eV −(−54.4 eV)
= 40.8eV ≈ 41eV
Similarly,
E3 − E1 = −6.04 eV −(−54.4 eV)
= 48.36eV
E4 − E1 = −3.4 eV −(−54.4 eV)
= 51eV
E3 − E2 = −6.04 eV −(−13.6eV)
= 7.56eV
and so on.
But note that E2 – E1 ≠ 42 eV, E3 – E1 ≠ 42 eV, E4 – E1 ≠ 42 eV and E3 – E2 ≠ 42 eV.
For all possibilities, no difference in energy is an integer multiple of photon energy. Hence, photon A is not absorbed by this atom. But for Photon B, E4 – E1 = 51 eV, which means, Photon B can be absorbed by this atom.
(e) Since total energy is equal to negative of kinetic energy in Bohr atom model, we get
KEn = −En = −( − 54.4/n2 eV)
= 54.4/n2 eV
Potential energy is negative of twice the kinetic energy, which means,
Un = −2KEn = −2(54.4/n2 eV)
= −108.8/n2 eV
For a ground state, put n =1
Kinetic energy is KE1 = 54.4 eV and Potential energy is U1 = –108.8 eV
Chapter 12 from the Textbook
by Subhas C Chakra
ATOMS
THOMSON'S ATOMIC MODEL
This model suggests an atom to be a tiny sphere of radius, containing the positive charge. The atom is electrically neutral. It contains an equal negative charge in the form of electrons, which are embedded randomly in this sphere, like seeds in a watermelon.
This model failed to explain
large scattering angle of α-particle
origin of spectral lines observed in the spectrum of hydrogen atom
ALPHA-PARTICLE SCATTERING AND RUTHERFORD'S NUCLEAR MODEL OF ATOM
In the Rutherford α- particle scattering experiment a very fine beam of α-particles passes through a small hole in the lead screen. This well collimated beam is then allowed to fall on a thin gold foil. While passing through the gold foil, α-particles are scattered through different angles. A zinc sulphide screen is placed out the other side of the gold foil, this screen is movable, so as to receive the α-particles, scattered from the gold foil at angles varying from 0 to 180°. When an α-particle strikes the screen, it produces a flash of light.
FINDINGS
Most of the α-particles went straight through the gold foil and produced flashes on the screen as if there were nothing inside gold foil. This suggests that the most part of the atom is empty.
Few particles collided with the atoms of the foil which have scattered or deflected through considerable large angles. Very few particles even turned back towards the source itself.
CONCLUSIONS
The entire positive charge and almost whole mass of the atom is concentrated in a small centre called a nucleus.
The electrons revolving around the nucleus could not deflect the path of α-particles. This suggests that electrons are very light.
In 1911 Rutherford, proposed a new type of model of the atom. According to this model, the positive charge of the atom, instead of being uniformly distributed throughout a sphere of atomic dimension, is concentrated in a very small volume at its centre. This central core, called nucleus, is surrounded by clouds of electrons that makes the entire atom electrically neutral.
According to Rutherford scattering formula, the number of
α-particles scattered at angle θ by a target,
N ∝ cosec4 (θ/2)
Impact parameter
Distance of closest approach
RESULT OF RUTHERFORD SCATTERING EXPERIMENT
Nucleus is a central, massive, positively charged core, its size is of the order of 10–15 m. The number of electrons surrounding the nucleus is such that the atom is electrically neutral.
Unit for nuclear dimension measurement : 1 fermi = 10–15m.
BOHR’S ATOMIC (HYDROGEN ATOM) MODEL
In 1913 Bohr gave his atomic theory primarily to explain the spectra of hydrogen and hydrogen-like atoms. His theory contained a combination of views from Plank’s quantum theory, Einstein’s photon concept and Rutherford model of atoms. The Bohr theory can explain the atomic spectra of hydrogen atoms and hydrogen-like ions such as He+, Li2+, Be3+...(one electron ions). But his theory failed to explain the spectra of more complex atoms and ions.
BASIC POSTULATES OF BOHR’S MODEL
The electron moves in circular orbits around the nucleus under the influence of coulombic force of attraction between the electron and the positively charged nucleus (as shown in figure below).
Bohr’s model of hydrogen atom
The electron rotates about the nucleus in certain stationary circular orbits, for which the angular momentum of electron about the nucleus is an integral multiple of, where h is plank’s constant
i.e., Angular Momentum, ...(1)
(where n = 1, 2, 3......... principal quantum number)
When the electron is in one of its stationary orbits, it does not radiate energy, hence the atom is stable. These stationary orbits are called allowed orbits.
The atom radiates energy when the electron “jumps” from one allowed stationery state to another. The frequency of radiation follows the condition
hν = Ei – Ef ...(2)
Where Ei and Ef are total energies of initial and final stationary states. This difference in energy (Ei -Ef) between two allowed stationary states is radiated/absorbed in the form of a packet of electromagnetic energy (hν - one photon of frequency ν) called a photon.
Now we calculate the allowed energies of hydrogen atom,
For moving an electron in a circular orbit the required centripetal force is provided by the coulomb force of attraction which acts between nucleus [Ze+, here Z = 1 (atomic number) for hydrogen atom] & electron (e–),
i.e., ...(3)
where is electrostatic constant & εo is permittivity of free space.
Eliminating v from eqn. (1) and (3) we obtain radius of nth orbit
(where n = 1, 2, 3 .....) ...(4)
Equation (4) gives the radii of various orbits (have discrete values).
The smallest radius (also called Bohr radius) corresponds to n = 1 is
...(5)
⇒ r = 0.529 n2 Å for hydrogen atom and
r = 0.529 × for hydrogen like ions.
From equation (4) & (1) we obtain,
Velocity of electron in nth state
or (for hydrogen atom ) ...(6)
for hydrogen like ions
The total energy of electron is given by
E = K.E. + P.E. = Kinetic energy + Potential energy
...(7)
(Allowed energy state)
After substituting numerical values in eqn.(7), we obtain
(for hydrogen atom) ...(8)
(for hydrogen like ions)
The lowest energy state, or ground state, corresponds to n = 1 is
The next state corresponds to n = 2 i.e., first excited state has an energy, E = –3.4 eV
LIMITATIONS OF BOHR'S MODEL
It could not explain the spectra of atoms containing more than one electron.
There was no theoretical basis for selecting mvr to be an integral multiple of .
It involved the orbit concept which could not be checked experimentally.
It could not explain Zeeman & Stark effect and fine lines of spectra.
It was against de-Broglie concept and uncertainty principle.
POINTS TO REMEMBER
Total energy of electron = – Kinetic energy
The reference level for potential energy has been taken as infinity
The energy gap between two successive levels decreases as the value of n increases
The radius difference between the successive orbit (or shells) increases as the value of n increases
The velocity of electrons around the nucleus goes on decreasing as n increases
The time period of the electron in an orbit
Maximum number of spectral lines that can be emitted when an electron jumps from nth orbit is
ENERGY LEVELS AND THE LINES SPECTRA OF HYDROGEN ATOM
An energy level diagram of the hydrogen atom is shown in figure. The upper most level corresponding to n→, represents the state for which the electron is completely removed from the atom.
Some transitions for the Lyman, Balmer & Paschen series are shown. The quantum numbers are at left & energies of levels are at right.
E = 0 for r = (Since n = )
If the electron jumps from allowed state ni to allowed state nf, then frequency of emitted photon is given by
...(1)
and the wavelength of emitted photon is
for hydrogen atom ...(2)
and ( for H-like atoms)
where R = 1.096776 × 107m–1 is known as Rydberg constant. By using this expression we can calculate the wavelengths for various series (Lyman, Balmer...) in hydrogen spectrum, i.e.
Lyman series ni = 1 & nf = 2, 3, 4...............
Balmer series ni = 2, & nf = 3, 4, 5...............
Paschen series ni = 3 & nf = 4, 5, 6..............
Brackett series ni = 4 & nf = 5, 6, 7...............
P fund series ni = 5 & nf = 6, 7, 8...............
First three series of hydrogen atom are shown in figure.
But in practice, the value of Rydberg constant varies between and R
This is because in above calculations we assumed that an electron revolves around a massive fixed nucleus of mass M. But in reality, the electron and nucleus each revolve round their common centre of mass i.e., the motion of the nucleus cannot be ignored. The correction for nuclear motion amounts to replacing electronic mass m by reduced mass μ which is defined as
...(3)
So total energy by taking this correction is
...(4)
If we are dealing with hydrogen like ions such as – He+, Li2+, Be3+, Be4+ (one electron ions), each can be considered as a system of two charges, the electron of mass m & charge –e & nucleus of mass M and charge +Ze, where Z is atomic number. The radii of circular orbits for these one electron ions can be written as
(n = 1, 2, 3............) ...(5)
and the allowed energies are given by
(n = 1, 2, 3.........) ...(6)
WAVELENGTH LIMITS IN VARIOUS SPECTRAL SERIES OF HYDROGEN ATOM
For Lyman series (lies in ultraviolet region)
Here
For Balmer series (lies in visible region)
Here
For Paschen series (lies in infrared region)
Here
For Brackett series (lies in infrared region)
Here
For p-fund series (lies in infrared region)
Here
POINTS TO REMEMBER
The first line of Lyman series is when electron jumps from 2 → 1, It is also called α–line
The second line of lyman series is when electron jumps from 3 → 1, It is also called β–line
The limiting line of lyman series is when electron jumps from ∞ → 1
Energy of electrons in different orbits in an atom varies inversely with the square of the number of orbits. So, energy of electrons increases (decreases in negative) as the orbit becomes higher.
If energy of a particular orbit is E for H-atom then its value for a H-like atom with atomic number Z is given by E' = E × Z2.
If the radius of a particular orbit of H-atom is R then its value for a H-like atom is given by
If velocity of an electron in a particular orbit of H-atom be v then its value for H-like atom is given by
v'= v × Z.
If kinetic energy and potential energy of an electron in a particular orbit of H-atom be T and V respectively then their corresponding values for H-like atom are given by
T' = T × Z2 and V' = V× Z2.
COMMON DEFAULT
🗶 Incorrect. Bohr's formula for spectral lines does not differentiate between isotopes. For example the first line of Lyman series in hydrogen and deuterium will have same wavelength because





















No comments:
Post a Comment